|
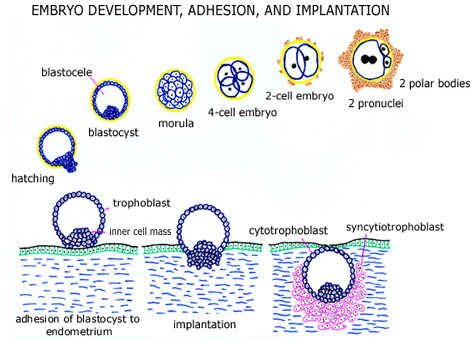
Immediately following fertilization, cell division is initiated. Two (male and female) pronuclei form and are visible within the egg’s cytoplasm within roughly 20 hours of fertilization. The fertilized egg (pre-implantation embryo) then divides into 2 cells, 4 cells, 8 cells, etc. A morula (solid mass of cells = blastomeres) develops about 3-4 days after fertilization, and a cavity (blastocele) subsequently forms within this mass of cells to form a blastocyst (about 4-5 days after fertilization). The pre-implantation embryo normally leaves the fallopian tube to enter the uterine cavity at the late morula or blastocyst stage of (embryonic) development.
The blastocyst normally “hatches” from the zona pellucida (shell) within the uterine cavity and the thickened region of the blastocyst (the “inner cell mass”) adheres to and implants into the endometrial lining. The embryo’s initial outer shell of trophoblast cells is called cytotrophoblast and following implantation some of these (cytotrophoblast) cells fuse to form multinucleated placental cells called syncytiotrophoblast. Cytotrophoblast and syncytiotrophoblast cells have very different functions and hormone production, each important in the development of a normal pregnancy.
The endometrial lining is prepared for implantation following ovulation through a series of molecular events that are not fully understood. It appears that the primary hormone involved in these post-ovulatory changes is progesterone. The uterine (endometrial) lining is “receptive” to an embryo only during a short window of time, typically during cycle days 18-22 of a 28 day natural cycle. This window of uterine receptivity appears to be shorter (about 3 days long) during cycles of controlled ovarian hyperstimulation.
Human embryo implantation normally occurs (in a 28 day natural cycle) about 5-7 days after fertilization. The fertilized egg remains in the fallopian tube for the initial 4-5 days following fertilization, a late morula or blastocyst stage embryo then enters the uterine cavity, and the blastocyst remains within the “uterine secretions” for 1-3 days prior to hatching and implantation.
Apposition and adhesion of the pre-implantation embryo to the uterine endometrial surface is mediated by a variety of cell adhesion molecules (including integrins and selectins) and extracellular matrix proteins (like fibronectin and laminin). These events are poorly understood and are currently an area of active medical research. During the implantation process, the developing embryo and trophoblast tissue invades the maternal (mother’s) blood supply within the uterine wall to establish a source of nutrition (and a way to excrete metabolic breakdown products). Invasion of the implanting embryo is at least partially mediated by metalloproteinases (such as collagenases and gelatinases, which degrade the extracellular matrix during implantation), which are regulated by the actions of other molecular messengers (including plasminogen activators and cytokines). The molecular events that occur during implantation are also an area of active medical research.
|

|